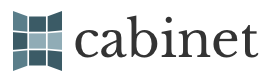Newton's Alchemical Sublimations
External links
Commentary
Isaac Newton actively experimented with alchemy over a span of some four decades, from his first serious engagement with the sciences in the mid-1660s up until the first decade of the eighteenth century when he became President of the Royal Society and was knighted. Throughout this period, Newton kept careful records of his alchemical experiments and copied numerous books and manuscripts on the subject, many of which are available here. He was heavily influenced by such popular alchemical authors as the Polish courtier and mining expert Michael Sendivogius, the wandering German adept Johann de Monte-Snyders, and the English-speaking chymist Eirenaeus Philalethes, who was in reality George Starkey, a native of Bermuda and the most famous scientist of the British North American colonies before Benjamin Franklin.
Despite Newton’s careful reading of these and numerous other alchemical writers, his own experimental practice deviated from theirs in striking ways, however. Instead of focusing on saltpeter like Sendivogius or on antimonial dendrites like Starkey, Newton employed a complicated series of processes intended to break minerals and metals into their most primitive constituents by making them increasingly volatile.
His laboratory notebooks repeatedly describe a series of protocols intended to achieve this goal, which can be simplified in the following fashion. Typically Newton employs a solution of sal ammoniac in aqua fortis (nitric acid) to produce a sort of aqua regia (nitric and hydrochloric acid). He then reacts this with stibnite (ore of antimony), which yields a solution that he usually calls “liquor of antimony” along with a yellowish “calx” or precipitate. He then reacts the “liquor of antimony” with a metal or mineral, which yields another solution and another precipitate. At this point, he either focuses on the solution, which he often evaporates and crystallizes to yield what he calls a “vitriol,” or else he focuses on the precipitate. In the latter case, he frequently dries the precipitate and then sublimes it, usually with a sublimate that has already been prepared either explicitly from stibnite and sal ammoniac or with “sophic” sal ammoniac, a proprietary material whose exact production he never describes.
Replications of Newton’s experiments performed at Indiana University have shown that his sublimations produced complex double salts of the metals and metalloids that he subjected to these processes. The present illustration, photographed while the apparatus was still hot, shows the different bands of colors produced by diverse sublimates that managed to infiltrate the pores of the inverted refractory crucible that served as their receptacle. Newton did in fact succeed in rendering metals volatile in this fashion, which encouraged his belief that he was on the way to discovering the philosophers’ stone. Far from being a sterile dead end, his alchemy was producing new and interesting compounds with properties that were very unlike the materials of contemporary chymistry.
Credits: William R. Newman (April 2022)